Patent System Q&A
8. REGISTRATION & MAINTENANCE FEES
- 1-3rd year: JPY12,900 + JPY900 per claim. (These figures are for the 3 years in total. Also serves as the registration fee.)
- 4-6th year: annually, JPY10,300 + JPY800 per claim (each year).
- 7-9th year: annually, JPY24,800 + JPY1,900 per claim (each year).
- 10-25th year: annually, JPY59,400 + JPY4,600 per claim (each year).
- Municipal Tax --> non-work-related income is less than JPY 1.5 million.
- Income tax --> income less than JPY 2.5 million.
- Business tax --> real estate income and business income is less than JPY 2.9 million.
- Official Fee: JPY1,000 per case
- Admin Fee
- Power of Attorney original executed document, not electronic or photocopy)
- A statement called a “Partial waiver of Patent Right” indicating which claims you wish to waive
What is the scope, timeline and cost for patent registration and maintenance fees in Japan?
Applicants are not required to pay maintenance (annuity) fees until an application is granted.
Registration
Annuities for the 1st to 3rd year of granted patents also serve as the patent registration fee. Yrs1-3 have to be paid together, and within 30 days from receiving the decision to grant* — only then can a certificate of a patent be issued. After this payment, the registration takes around 14 to 21 days to be recorded.
Certificate of Patent
1. Electronic Certificates
Effective April 1, 2024, the JPO issues electronic certificates for patents, utility models, designs, and trademarks in most cases only, rather than traditional paper certificates (design and trademark registrations via the Hague or Madrid systems will still be paper-based). It usually takes around 2-3 weeks to receive the electronic certificate.
Electronic certificates are issued only once. Reissues of the certificate are only available in paper format and can be requested for JPY 4,600, regardless of the reason necessary for the reissue.
No interim changes are possible to the certificate, which will contain the same information as at the time of the original issue.
Note that the certificate can still be issued in paper format instead of electronically through a Japanese IP representative if the rights holder requests.
2. Paper Certificates reissue
As of January 1, 2025, it is also possible to request the reissue of an original paper patent certificate, regardless of the reason (previously, reissues were only possible for damaged or lost certificates). The requirement to return damaged paper certificates also no longer applies from this date. The same reissue fee as above applies (JPY4,600).
The content on the reissued patent certificate cannot be altered from the time of the original issuance.
In cases where there is more than one rights holder, reissued certificates can be requested for all rights holders; however, separate reissue fees will apply (JPY 4,600 x X).
It takes approximately one month for the applicant’s Japanese representative to receive a reissued certificate after the request. The paper certificate can then be forwarded via EMS FedEx, etc., to the applicant overseas for a small handling fee. This will include the original certificate, our English translation (other languages available) and information on the annual patent fees necessary to maintain the patent right.
Maintenance from 4th year
Maintenance (annuity fees) for the 4th year onwards should be paid by the anniversary of the day the patent was issued in the year up to which fees have already been paid. Fees from the 4th year onward can be paid per year or otherwise.
*Note: it is possible to obtain 30-day extensions for paying the registration fee for a small fee. This extension will also apply to filing divisional applications.
When will I know if payment has been made?
The JPO will issue a payment receipt about two months after the date on which payment was received.
May I request a refund for an annuity payment if I wish to abandon my patent?
Once a maintenance fee payment has been made, you may not cancel it. The only "refunds" available are: when you have overpaid; when the patentee is eligible for annuity fee reduction (restricted to the first 10 years of annuities), See 8.2 Maintenance Fee Reduction; or related to an invalidation, etc.
Does the JPO issue documents when an IP owner pays a maintanence fee?
The JPO will issue a "Notice of Renewal" to the owner when payment has been made. Additionally, the owner can obtain a copy of the "patent (or trademark, etc.) register", which contains details of all rights of the patent, and includes dates, addresses and renewals(paid annuities), etc.. Renewals are recorded in this register.
The JPO retains the original register, but owners can request a digital (PDF) or physical (printed paper) copy of (with updated renewal information). You can also request that this be certified by the JPO.
It takes around 3–4 weeks to get the physical copy, or around 2 weeks for the digital copy only.
OFFICIAL FEES
General official fees for maintenance (annuity) fees are as follows (effective from April 1, 2022):
KIPB charges a small admin fee for each annuity submission.
Is there any way to reduce maintenance fees?
JPO Fee reduction scheme
The JPO has an official fee reduction scheme for various SMEs, individuals, start-ups and research institutes (universities), etc. The reduction applies to examination and annuity fees for years 1 to 10. If a patent right holder qualifies, annuity fees will be reduced (to 1/2 or 1/3 the standard fee) depending on which requirements are satisfied, and the type of business.
Does the fee reduction scheme apply for individual inventors or small business owners?
The scheme is open to “sole proprietors” and “individuals (with modest means)”.
In Japan, a “Sole proprietor” refers to a person who employs 20 people or less (5 or less if commercial or service business), as evidenced by the submission of a notice of commencement of a commercial business to the tax office.
Foreign organizations equivalent to the Japanese “sole proprietor” can also qualify for the fee reduction scheme.
If you qualify, official examination fees and patent fees are reduced to 1/2 or 1/3 of the standard fees.
Qualifying as an "individual (with modest means)" involves certain conditions, such as exemption from municipal tax, income tax or business tax.
If you qualify, official Examination request fees and patent fees are either exempted or reduced to 1/2 standard fees depending on the qualification.
Please contact us if you think you or your client might qualify.
*The reduction scheme also applies to examination fees during prosecution.
Reducing the number of claims after grant
It is also possible to reduce (abandon) one or more claims in a Japanese patent after patenting. Since annuity fees are based in part on the number of granted claims, this can work to reduce annuity fees. The costs for reducing claims are not on a per-claim basis, but per case - so you can reduce any number of claims for the same fee.
See below:
We require the following documents in order to make the change:
Please contact us if you wish to do this.
Note that the claim reduction applies to annuity fees from Yr4 (it is not possible for newly allowed patents.) This means the registration fee (i.e., annuities for Yrs1-3) will have to be paid with the full per claim fee included.
Note also that the claim reduction is simple deletion: it is not possible to make substantial amendments, like combining multiple claims into a single claim.
Is there a grace period after the due date for an annuity payment?
Yes. The patent can be maintained by paying a double maintenance fee within 6 months after the due date.
You do not need to request the 6-month grace period, which will be entered automatically upon non-payment by the deadline.
Can I restore my patent if I miss an annuity payment deadline and grace period?
Yes, if you can show the failure to pay by the deadline + 6-month grace period was due to an "unintentional" error (including human error and failure of managerial oversight), you can restore your patent right by paying the patent fee + grace period surcharge + recovery request surcharge within two months of the date on which the unintentional reason ceased to exist (provided this is within one year after the expiry of the 6-month grace period.)
Effectively, this means you have 1.5 years after the original statutory deadline to maintain the patent if you follow the necessary procedures as set out above.
This rule is effect from April 2023, so any missed payment deadlines before that date will be subject to the previous (stricter) recovery rules
For more details on the recovery system in Japan, see OTHER QUESTIONS, Q4: Recovery of Application After Missed Deadline
How long is a patent right in Japan?
Patent rights in Japan start from patent registration and last for 20 years. However, this 20-year term is calculated from the filing date (or the international filing date for PCT filings) not the registration date, provided regular annuity fees are paid (see 8.1 Registration & Maintenance Fees/Scope).
Are term extensions possible?
Generally, the 20-year term cannot be adjusted. However, term extensions can be requested for certain patents.
- For instance, when there is a period of time during which the patented invention could not be worked due to other legal restrictions (e.g. for pharmaceutical and agrochemical related patents which require safety approval before marketing). The patent term may be extended by up to five years.
- More recently, patent terms of patents that have been registered “late” due to JPO delays during prosecution may also be extended.
(see 8.5 Patent Extension System).
Patent Term of Divisional Applications
Since divisional applications have the same effective filing date as the parent (or grandparent) application, the patent term for the divisional also expires 20 years from the filing date of the parent application (or 20 years from the international filing date of the parent if the parent is a PCT national phase application).
The above extensions apply to both parent and divisional applications if eligible.
Is there a patent extension system in Japan?
Yes. There are currently two ways to extend a patent term beyond the standard 20-year term in Japan (calculated from the filing date or from the international filing date for PCT derived patents.) The first compensates for a “late” allowance of a patent due to delays in examination at the JPO. The second compensates for the extra time taken to obtain regulatory marketing approval for certain pharmaceutical and agrichemical patents.
Patent term extensions due to “late” allowance by the JPO
Patent term extensions can be requested for patent applications filed on or after March 10, 2020, if the JPO is “late” in allowance of the application. “Late” allowance is defined as 5 or more years after filing or 3 years after a Request for Examination (whichever is later.)
All patented inventions are eligible (regardless of whether or not they contain government-regulated substances, see below.)
How is the extension calculated?
The period of extension is calculated based on the time elapsed after these periods (subtracting the periods for responding to office actions, extensions for responding to office actions and appeals etc.) The request for term extension should usually be made within 3 months of registration.
Patent term extensions for pharmaceutical and agrichemical patents
Patent extensions are also available for certain pharmaceuticals and agrochemical patents that require regulatory approval before the patented product/substance can be marketed. The patent life of such inventions can be extended to compensate for the time taken whilst waiting for this approval.
This is an important way of preventing the market entry of generics and maintaining the patent holder’s exclusivity to profit from a new drug.
How is the extension calculated?
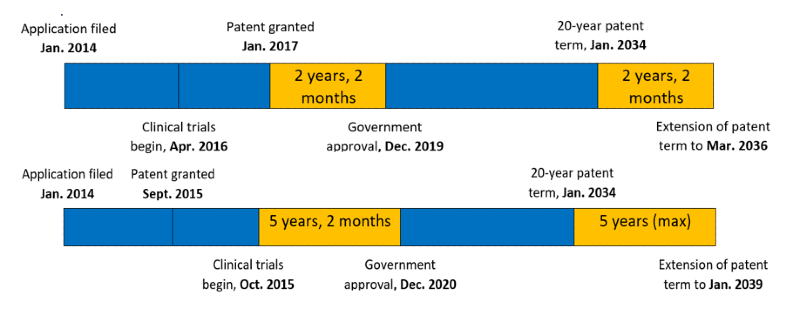
The time period of the extension is equal to “the period during which the patented invention was unable to be worked” whilst waiting to receive regulatory authorization (Japanese Pharmaceutical and Medical Device Law).
This period is calculated from either
(i) the date clinical trials started (submission of trial plan), , or
(ii) the day when the patent was registered
(whichever is later)
to
(iii) one day prior to the date on which the patentee receives approval for the drug in Japan.
The available extensions are for a maximum of 5 years even if this (i)(ii) to (iii) period is longer.
Examples of this calculation:
Two or more term patent extensions may be possible for a single patent if multiple marketing approvals are obtained. Also, multiple patents may be extended independently based on a single marketing approval of a product if they all pertain to the same single marketing approval.
Note also that “Paediatric extensions” with respect to patent terms are not available in Japan at the time of writing.
Another way to maintain exclusivity of new drugs
Note that there is another way owners of new pharmaceutical products may protect against market entry of third-party generic drugs in Japan. See 8.6 Market exclusivity derived from the "re-examination" of a drug after market approval for an explanation.
For more information on patent extension procedures, etc., please refer to the following:
(i) JPO: Patent Term Extensions;
(ii) Japan Pharmaceutical Manufacturers Association’s (JPMA) booklet on Pharmaceutical Regulations in Japan, Chapter II, 3.22 Patent System (p.31).
Does Japan have a market (data) exclusivity scheme following grant of pharmaceutical drug marketing rights?
Whilst there is no established regulatory marketing / data exclusivity for pharmaceutical or biological drugs in Japan per se, there is a "post-marketing surveillance system/period" (PMS) (i.e., a re-examination period) that may operate as de facto post-marketing exclusivity that can effectively delay market entry of generics, etc.
Re-examination
PMS involves a period of re-examination of a new drug that is set by the Japanese drug regulatory body (Japan Pharmaceuticals and Medical Devices Agency - PMDA) after the marketing approval (MA) of the drug. During this period the regulatory body will collect further data from the holder of the approved drug and re-examine its safety and efficacy. Re-examination is necessary due to limitations to the time for / scope of supporting data supplied to the body for the initial MA stage.
During this re-examination period, third parties seeking marketing approval for a generic drug (with identical active ingredients, etc):
A) cannot access the data supplied to the regulatory body by the MA holder, and
B) must provide data/materials that is at least as comprehensive as for the approved drug (this requirement is relaxed after the re-examination period).
This effectively acts as a barrier to entry for a generic drug (period of exclusivity).
Time periods for different drugs
The stipulated time periods for re-examination from the date of approval depend on the type of drug (in general).
Thus;
• orphan drugs - 10 years after the date of approval;
• drugs with new active ingredients - 8 years after the date of approval;
• drugs with new routes of administration - 6 years after the date of approval;
• drugs with new combinations, indications or dosages, etc - 4-6 years after the date of approval.
Effectively, a generic company is unable to commence marketing of their product until after this period.
Another way to maintain exclusivity of new drugs - 'patent term extension'
Patent term extensions are also available to owners of patented pharmaceutical products, which protect against market entry of third-party generic drugs in Japan. See 8.5 Patent Term Extension for an explanation.
What is the difference between 'patent term extension' and the 're-examination' system?
The two approaches both derive from the fact that third parties may file for marketing approval of a generic drug that is within the technical scope of a patent – this does not constitute infringement.
Thus, a patent term extension bars third parties from actually manufacturing, using or selling the generic drug during the term, whilst re-examination sets extremely onerous criteria for the generic to achieve marketing approval for sale during the period of re-examination.
----------------------------------------------------
Important: You should note that this is not KIPB’s specific area of expertise. We only include it here since many clients ask for this information.
You can find more information online in English at:
(i)"Pharmaceutical Administration and Regulations in Japan 2020" compiled by the Japan Pharmaceutical Manufacturers Association (JPMA). See particularly Chapter 4 on post-marketing surveillance of drugs.
(ii) The Japan Pharmaceuticals and Medical Devices Agency (PMDA).
Is it possible to amend claims after grant?
General amendments are not possible for granted applications. However, a patent holder (only) may request a “Trial for Correction” in order to correct/clarify the patented claims.
The scope of amendments (“corrections”) is rather limited, and the purpose is generally as a counter-action in anticipation/advance of an invalidation trial or opposition (see below).
Trial for Correction scope
Correction is limited to the following:
(i) Cancellation of claims;
(ii) Restriction of the scope of claims;
(iii) Correction of errors or incorrect translations;
(iv) Clarification of unclear descriptions (within the scope of the original specification); and
(v) Rewriting a claim citing another claim so that it does not cite the other claim.
(The corrections should not broaden the scope of the claimed inventions, and should remain within the scope of the original disclosure.)
Note also that fees for correction trials are relatively high for the scope that is possible. However, once filed, the examination is usually fairly quick (3 months or so)
Note also that corrections can also be made when an Invalidation Trial is demanded by a third party. Patent holders cannot request a separate correction trial during the period when an invalidation trial is still pending. A patent holder might therefore request a correction trial beforehand if they anticipate their registered claims etc. are at risk of invalidation.
The Divisional option
Patent holders wishing to pursue other claims based on the granted application may file divisional applications (as long as allowance was not via an Appeal). Divisionals can be requested within 30-day after allowance, but NOT after the registration fee has been paid.
Can Divisional applications be filed after allowance?
Divisional applications can be made after grant, provided the parent application was not granted via an appeal procedure. Divisionals can be requested within the same 30-day period for paying the registration fee (with the extensions), but NOT after the registration fee has been paid.
*Note that it is possible to obtain 30-day extensions for paying the registration fee for a small fee, which also effectively also applies to filing divisional applications.
When can I begin enforcing my patent against infringers?
Patentees can enforce against infringers as soon as the patent is registered. This includes filing a petition for preliminary or permanent injunction or lawsuit seeking damages. However, it is difficult to know the actual date of registration since the JPO does not notify patentees of this. In this instance, KIPB can monitor the JPO databases daily to see if registration has been completed, upon request.
Note also that you can a warning letter to a potential infringer before registration, but after publication of your application. Whilst you cannot sue for damages or request injunction before registration, the potential infringer may settle or negotiate. The court might also look on the letter favourably as evidence of infringement, and it may be easier to assert damages for the period before registration.



